Shape-shifting in the fight against cancer
Understanding changes in T cell metabolism offers new pathways in cancer treatment
Cells of the immune system have a metabolism that can be changed depending on functions and needs during an immune response. Researchers at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg were searching for signals that cause the these metabolic changes and now able to show that the structure of mitochondria is crucial for the regulation of the immune cell metabolism. Further, the study of the scientists in Freiburg describes that the shape of mitochondria can be tuned to enhance the ability of immune cells to recognize and destroy tumor cells. The results are published in Cell.
“Yin and Yang” of T cell fate. The shape of mitochondria controls T cell metabolism and immune reaction.
T cells are a subset of white blood cells, which can recognize and destroy virally infected or cancerous cells. In the last few years it has been shown that the engagement of particular metabolic pathways is critically important for immune cell fate. But it still remains unclear which signals drive T cells to preferentially emphasize a particular type of metabolism to establish their function and differentiation. Scientists at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg have now discovered that the morphology of mitochondria controls T cell metabolism, and further, can be tuned to enhance antitumor immunity.
Metabolism decisive for the immune response
It is already known that during an immune response T cells undergo extensive metabolic changes. For instance, to exert their functions, activated T cells, must shift from an energy-saving metabolism that supports quiescence and immune surveillance, to an anabolic and biosynthetic metabolism that supports T cell growth and effector functions. Thus, activated T cells switch their metabolism toward aerobic glycolysis and increase their consumption of glucose. However, in cancer, a growing tumor competes with T cells for glucose and other nutrients that both tumor cells and T cells need for their survival, but which T cells also require to mediate an effective antitumor response. Recent studies showed that the inability of T cells to effectively utilize aerobic glycolysis in this hostile tumor environment could sway the outcome of tumor progression.
Erika Pearce - How Does Metabolism Influence the Function of Different T Cell Types?
If an infection or cancer is cleared, most of the T cells that were engaged in the protective response die. However, a subset of T memory cells persists and provides critical long-term protection. Metabolism of T memory cells relies on effective fatty acid oxidation, which equips them to rapidly respond should an infection or cancer recur. In this case, failure to utilize fats can severely inhibit the formation of T memory cells.
“To understand why specific metabolic programs fail and impair T cell function we had to focus on the signaling mechanism that instructs metabolic programming,” says Erika Pearce, senior author and director at the Max Planck Institute in Freiburg.
Her team focused on the role of the mitochondrion, “the powerhouse of the cell”. This expression describes the main function of mitochondria, which are organelles that release energy from nutrients and act as essential hubs of metabolic activity.
Structure of the mitochondria determines metabolism
In addition to the numbers of mitochondria increasing or decreasing in a cell as energy requirements change, these organelles can also take on vastly different shapes and sizes. Mitochondria can exist as discrete, fragmented entities, or in long tubular networks. In their current study published in Cell, the scientists were able to show that the morphology and organization of the mitochondria underlie and enforce the characteristic metabolic phenotype of T cells.
“We found that activated effector T cells have fissioned mitochondria, while T memory cells maintain their mitochondria in fused networks” says Michael Buck, first-author of the study. To test if the structure of mitochondria controls metabolic programs and therefore regulates T cell responses, the team used different drugs to enforce mitochondrial fusion in activated T cells.
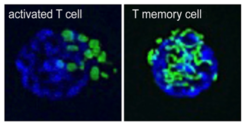
“Our data suggest that manipulating the structure of the mitochondria can have profound consequences that impact metabolism and ultimately cellular differentiation,” reports Erika Pearce. The scientists saw that activated T cells in which fusion had been promoted adopt T memory cell characteristics. The treated cells acquire enhanced longevity and the ability to control tumor growth. Concurrently, fission-promoting events remodel mitochondria in T cells and act as a signal to drive the induction of aerobic glycolysis, which is the characteristic metabolic program of activated T cells.
These new results by the researchers of the Max Planck Institute in Freiburg open many exciting avenues for future research. The authors envision that the signaling mechanism proposed to control distinct metabolic programs in T cells may be a target for therapy against human disease. “Proper metabolic programming in T cells is very important for effective immune responses. Targeting mitochondrial structure and organization with pharmacological agents seems to be a promising factor to control metabolic pathways,” says Erika Pearce. Michael Buck adds, “With a dedicated appreciation for the role of metabolism in T cells, one can think of targeting these mechanisms to prevent T cell dysfunction in a hostile tumor environment, or to generate more effective immunotherapies for cancer with fitter and better prepared T cells.”





